What does the recent ISO 14644-1:2015 update mean for pharmaceutical cleanroom classification?
In its first major revision since the original release in 1999, ISO 14644-1 was updated and approved as an International Standard in October 2015 and published in December 2015. This blog aims to provide a brief overview of the changes, but more importantly, how the changes affect the current PIC/S Guide to GMP for Medicinal Products – Annex 1 Manufacture of sterile medicinal products (PE009-12) and the average pharmaceutical cleanroom including:
- The application and uncertainty of ≥5.0µm particles in Grade A and Grade B environments for classification and monitoring to ISO 5
- The removal of the 95% UCL calculations
- The increase in sampling locations for most cleanrooms.
The 2015 updates to ISO 14644-2 relating to monitoring and reclassification should also be considered in conjunction with this blog.
ISO classification classes
The changes to ISO 14644-1 do not necessarily have an impact on the principles of classification however the basis for classification has been changed from ‘Classification by Formula’ (with a table to illustrate) to ‘Classification by Table’ (with a formula for intermediate sizes).
Below shows the illustrative table (derived from the formula) from ISO 14644-1:1999. The particular rows and columns that are of most interest in the pharmaceutical industry have been shaded. The table also highlights the particle concentrations that have been removed from the 2015 version of the standard in orange.
ISO 14644-1:1999 Table 1 – Selected airborne particulate cleanliness classes for cleanrooms and clean zones ISO 14644-1:1999 – Cleanrooms and associated controlled environments — Part 1: Classification of air cleanliness
ISO 14644-1:1999 – Cleanrooms and associated controlled environments — Part 1: Classification of air cleanliness
As a comparison, the new classification table from ISO 14644-1:2015 is shown below:
ISO 14644-1:2015 Table 1 – ISO Classes of air cleanliness by particle concentration

ISO 14644-1:2015 – Cleanrooms and associated controlled environments — Part 1: Classification of air cleanliness by particle concentration
“The reasons for these changes are related to the re-evaluation of the sensitivity of airborne particle counters and their ability to count small concentrations, and in the case of particles ≥5.0µm the collection efficiency has been reconsidered.” – Gordon Farquharson, Convenor ISO TC209 WG1 (Cleanrooms and associated controlled environments)
Based on the updated ISO class classification table in ISO 14644-1:2015, the following highlighted Annex 1 ‘maximum permitted particles’ now fall outside the ISO classification system and therefore should not be used for formal classification:
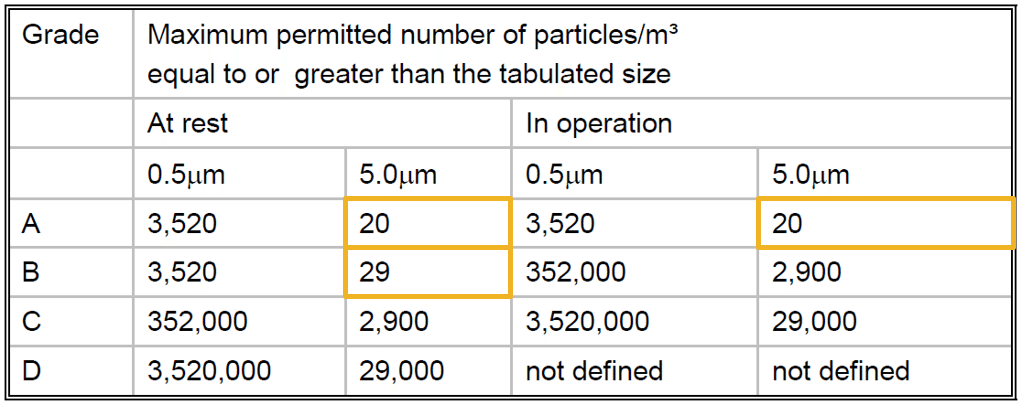
PIC/S Guide to Good Manufacturing Practice for Medicinal Products – Annexes – PE009-12 (Annexes) – 1 October 2015
This does not mean that they cannot be used in an appropriate way for measurement and reporting during a classification exercise or for measurement and assessment during real-time monitoring, they just should not be used as a classification attribute.
Macro-particle descriptor
The ISO 14644-1:2015 standard describes any particle with an equivalent diameter ≥5.0µm as a macro-particle. Where a regulatory agency demands consideration of these particles, the counting and sizing of these macro-particles is expressed using the M descriptor in the format:
ISO M (a; b); c
Where:
- is the maximum permitted concentration of macro-particles (expressed in particles/m3);
- is the equivalent diameter of the macro-particles;
- is the specified measurement method (typically Light Scattering Airborne Particle Counter (LSAPC)).
For example, the Grade A at rest concentration of 20 particles/m3 @ ≥5.0µm would be expressed as:
ISO M (20; ≥5.0 µm); LSAPC
The new M descriptor will be used to define the Annex 1 Grade A and Grade B and by using the existing ISO 14644-1 designation of airborne particle concentration (expressed as ISO Class number; occupancy state; considered particle size(s).
Grade A
- ISO 5; at rest, operational; ≥0.5µm
- ISO M (20; ≥5.0µm); at rest, operational; LSAPC
Grade B
- ISO 5; at rest; ≥0.5µm and ISO M (29; ≥5.0µm); at rest; LSAPC
- ISO 7; operational; ≥0.5µm, ≥5.0µm
Sampling, classification and re-classification
Annex 1 refers to ISO 14644-1 for the purpose of room classification including the number of sample locations and the sample size required. The 2015 version has seen an update to classification and sampling, the impact of which is an important change to the fundamentals of classification. The changes will require organisations to redefine their classification sampling plans and data evaluation.
ISO 14644-1:2015 has been simplified in regards to sampling and classification.
Key highlights of the changes:
- The 95% UCL (upper confidence limit) evaluation for 2-9 sampling locations has been removed.
- Each sampling location is to be evaluated separately and all sampling locations must pass for a cleanroom/zone to comply.
- The square root calculation used in ISO 14644-1:1999 to determine the minimum number of sampling locations has been replace with a look-up table. The table provides at least 95% confidence that at least 90% of the cleanroom or clean zone will comply with the ISO Class limit. This new method will likely result in an increase in sample points compared to the old square root calculation as shown in the chart below.
Note: A formula is still applicable for cleanrooms >1000m2.
- The location of sampling points will now be evenly distributed at representative locations while avoiding placement directly under a non-diffused HEPA filter supply air terminals in non-unidirectional airflow cleanrooms.

Looking ahead
March 2015 saw a concept paper released jointly by the European Medicines Agency (EMA) and PIC/S that detailed the updating of Annex 1. While we are not certain what the update to Annex 1 might contain at this stage, we anticipate it will accommodate the 2015 revision of ISO 14644-1 and ISO 14644-2. Will this mean the removal of the ≥5.0µm limit from the classification of Grade A and B rooms? Perhaps it will retain ≥5.0µm for real-time monitoring. These issues and other hot topics will be discussed in detail at the annual GMP and Validation Forum.


